Energetics of sodium–calcium exchanged zeolite A
Abstract
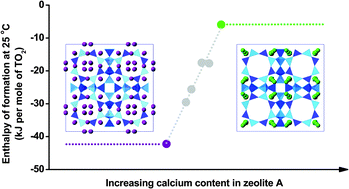
A series of calcium-exchanged zeolite A samples with different degrees of exchange were prepared. They were characterized by powder X-ray diffraction (XRD) and differential scanning calorimetry (DSC). High temperature oxide melt drop solution calorimetry measured the formation enthalpies of hydrated zeolites CaNa-A from constituent oxides. The water content is a linear function of the degree of exchange, ranging from 20.54% for Na-A to 23.77% for 97.9% CaNa-A. The enthalpies of formation (from oxides) at 25 °C are −74.50 ± 1.21 kJ mol−1 TO2 for hydrated zeolite Na-A and −30.79 ± 1.64 kJ mol−1 TO2 for hydrated zeolite 97.9% CaNa-A. Dehydration enthalpies obtained from differential scanning calorimetry are 32.0 kJ mol−1 H2O for hydrated zeolite Na-A and 20.5 kJ mol−1 H2O for hydrated zeolite 97.9% CaNa-A. Enthalpies of formation of Ca-exchanged zeolites A are less exothermic than for zeolite Na-A. A linear relationship between the formation enthalpy and the extent of calcium substitution was observed. The energetic effect of Ca-exchange on zeolite A is discussed with an emphasis on the complex interactions between the zeolite framework, cations, and water.

 Please wait while we load your content...
Please wait while we load your content...