Structure and dynamics of aqueous 2-propanol: a THz-TDS, NMR and neutron diffraction study†
Abstract
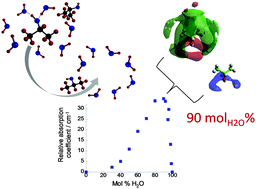
Aqueous liquid mixtures, in particular, those involving amphiphilic species, play an important role in many physical, chemical and biological processes. Of particular interest are alcohol/water mixtures; however, the structural dynamics of such systems are still not fully understood. Herein, a combination of terahertz time-domain spectroscopy (THz-TDS) and NMR relaxation time analysis has been applied to investigate 2-propanol/water mixtures across the entire composition range; while neutron diffraction studies have been carried out at two specific concentrations. Excellent agreement is seen between the techniques with a maximum in both the relative absorption coefficient and the activation energy to molecular motion occurring at ∼90 mol% H2O. Furthermore, this is the same value at which well-established excess thermodynamic functions exhibit a maximum/minimum. Additionally, both neutron diffraction and THz-TDS have been used to provide estimates of the size of the hydration shell around 2-propanol in solution. Both methods determine that between 4 and 5 H2O molecules per 2-propanol are found in the 2-propanol/water clusters at 90 mol% H2O. Based on the acquired data, a description of the structure of 2-propanol/water across the composition range is presented.


 Please wait while we load your content...
Please wait while we load your content...