Uranyl adsorption at solvated edge surfaces of 2 : 1 smectites. A density functional study†
Abstract
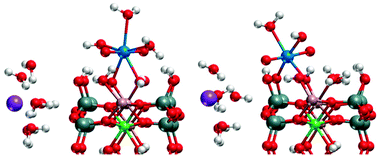
We systematically studied the adsorption of uranyl(VI) on two common edge surfaces, (010) and (110), of 2 : 1 smectite clay minerals, using standard periodic DFT models. To describe various types of permanently charged clay minerals, we introduced charged defects into the initially neutral layer of pyrophyllite, cation substitutions in tetrahedral (beidellitic) and octahedral (montmorillonitic) sheets. Comparing uranyl(VI) species at various sites of these two types of surfaces, we found that structural parameters of such adsorption complexes are essentially determined by the surface chemical groups forming the adsorption site, not by the type of the clay mineral. Even for sites involving a substituted cation we noticed only a weak effect of the substitution on the geometric parameters. Geometry optimization resulted in adsorbed uranyl or uranyl hydroxide, with coordination numbers of 4 or 5. However, in most cases the same species was determined on the same type of site, independent of the substitutions. Optimization of adsorbed uranyl leads to hydrolysis at sites close to a AlOH−1/2 surface group, resulting in uranyl monohydroxide as adsorbate and protonation of the AlOH−1/2 group. While most species are equatorially five-coordinated, coordination 4 is preferred when uranyl adsorbs on mixed AlO(H)–SiO(H) sites. Calculated formation energies of surface complexes do not single out a preferred species or site, but point to an equilibrium of several species. Comparison to experiment and consideration of pH conditions suggests AlOHOH and AlOH–SiO sites of (010) surfaces and AlOmOH, SiOOm, and AlOH–SiO sites of (110) surfaces as most probable for uranyl adsorption.

 Please wait while we load your content...
Please wait while we load your content...