Chiral recognition and atropisomerism in the sevoflurane dimer†
Abstract
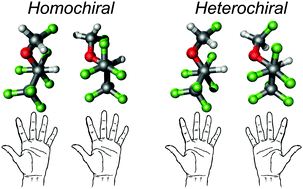
We have examined the stereoselectivity of molecular recognition between two molecules of the anesthetic sevoflurane using broadband rotational spectroscopy. The transient axial chirality of sevoflurane is revealed upon the formation of the dimer, as two different diastereoisomers made of either homo- or heterochiral species are detected in a supersonic jet expansion. The conformational assignment was confirmed by the observation of eighteen different isotopologues in natural abundance (all possible 13C's and two 18O species of the homochiral form). The two clusters are formed in practically equal proportions (1.1 : 1), probably due to their similar hydrogen bonding topologies. In both clusters the complex is stabilized by a primary C–H⋯O hydrogen bond, assisted by weak C–H⋯F interactions. This intermolecular binding regime is characterized by a mixture of electrostatic and dispersive interactions, midway between classical hydrogen bonds and van der Waals clusters.


 Please wait while we load your content...
Please wait while we load your content...