Adsorption of poly acrylic acid onto the surface of calcite: an experimental and simulation study†
Abstract
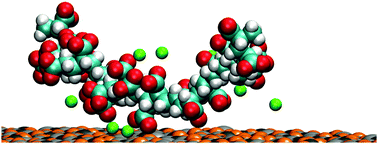
Macromolecular binding to minerals is of great importance in the formation of biofilms, and carboxylate functional groups have been found to play a pivotal role in the functioning of these macromolecules. Here we present both fluorescence time-resolved anisotropy measurements and simulation data on the conformational behaviour and binding of a poly acrylic acid polymer. In solution the polymer exhibits a pH dependent behaviour, with a coiled conformation at a low pH and extended conformation at higher pH values. The polymer is readily adsorbed on the surface of calcite, preferring to bind in an extended conformation, with the strength of the adsorption dependent on the pH and presence of counter ions. We discuss the reasons why the calculated adsorption free energy differs from that obtained from a Langmuir isotherm analysis, showing that they refer to different quantities. The enhanced binding of the extended conformations shows the importance of flexibility in the binding of macromolecules.

 Please wait while we load your content...
Please wait while we load your content...