A molecular dynamics study of model SI clathrate hydrates: the effect of guest size and guest–water interaction on decomposition kinetics†
Abstract
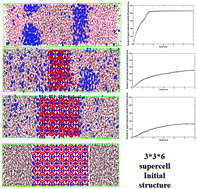
One of the options suggested for methane recovery from natural gas hydrates is molecular replacement of methane by suitable guests like CO2 and N2. This approach has been found to be feasible through many experimental and molecular dynamics simulation studies. However, the long term stability of the resultant hydrate needs to be evaluated; the decomposition rate of these hydrates is expected to depend on the interaction between these guest and water molecules. In this work, molecular dynamics simulation has been performed to illustrate the effect of guest molecules with different sizes and interaction strengths with water on structure I (SI) hydrate decomposition and hence the stability. The van der Waals interaction between water of hydrate cages and guest molecules is defined by Lennard Jones potential parameters. A wide range of parameter spaces has been scanned by changing the guest molecules in the SI hydrate, which acts as a model gas for occupying the small and large cages of the SI hydrate. All atomistic simulation results show that the stability of the hydrate is sensitive to the size and interaction of the guest molecules with hydrate water. The increase in the interaction of guest molecules with water stabilizes the hydrate, which in turn shows a slower rate of hydrate decomposition. Similarly guest molecules with a reasonably small (similar to Helium) or large size increase the decomposition rate. The results were also analyzed by calculating the structural order parameter to understand the dynamics of crystal structure and correlated with the release rate of guest molecules from the solid hydrate phase. The results have been explained based on the calculation of potential energies felt by guest molecules in amorphous water, hydrate bulk and hydrate–water interface regions.

 Please wait while we load your content...
Please wait while we load your content...