Interactions of CO2 with various functional molecules
Abstract
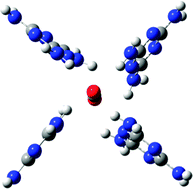
The CO2 capturing and sequestration are of importance in environmental science. Understanding of the CO2-interactions with various functional molecules including multi-N-containing superbases and heteroaromatic ring systems is essential for designing novel materials to effectively capture the CO2 gas. These interactions are investigated using density functional theory (DFT) with dispersion correction and high level wave function theory (resolution-of-identity (RI) spin-component-scaling (scs) Möller–Plesset second-order perturbation theory (MP2) and coupled cluster with single, double and perturbative triple excitations (CCSD(T))). We found intriguing molecular systems of melamine, 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), 7-azaindole and guanidine, which show much stronger CO2 interactions than the well-known functional systems such as amines. In particular, melamine could be exploited to design novel materials to capture the CO2 gas, since one CO2 molecule can be coordinated by four melamine molecules, which gives a binding energy (BE) of ∼85 kJ mol−1, much larger than in other cases.

 Please wait while we load your content...
Please wait while we load your content...