Self-healing dynamic bond-based rubbers: understanding the mechanisms in ionomeric elastomer model systems†
Abstract
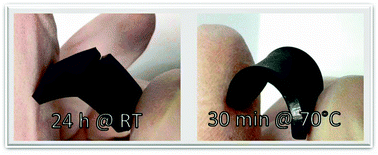
While it is traditionally accepted that the chain interactions responsible for the elastic response in an elastomeric network are ideally permanent and instantaneously active, the ongoing investigation of self-healing materials reveals that the introduction of self-healing properties into elastomers requires high mechanical integrity under dynamic load conditions, while on long timescales (or at extended temperatures), the chain and bond dynamics must allow for an intrinsic repair of micro cracks occurring during operation and aging. Based on an acrylate-based amorphous ionomer model system with pendant carboxylate groups allowing the systematic variation of the composition and the nature of the counter ion, we demonstrate the interrelation between the morphological, thermal, and mechanical properties, and identify the prerequisites and tools for property adjustment and optimization of self-healing efficiency. While the ion fraction is directly related to the effective network density and elastic performance, the crossover frequency between viscous and elastic behavior is influenced by the nature of the counter ion. In order to achieve reliable elastic response and optimal damage repair, the ion fraction in these systems should be in the range of 5 mol% and the chain dynamics should be appropriate to allow for excellent self-healing behavior at moderate healing temperatures.

 Please wait while we load your content...
Please wait while we load your content...