Sodium-ion diffusion mechanisms in the low cost high voltage cathode material Na2+δFe2−δ/2(SO4)3
Abstract
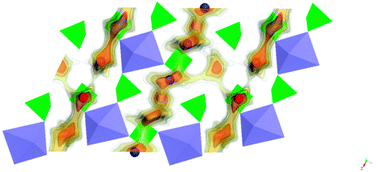
Bond-valence site energy modelling, classical molecular dynamics and DFT simulations were employed to clarify Na+ ion migration in monoclinic Na2+δFe2−δ/2(SO4)3, the recently reported first representative of a new promising class of alluaudite-type high voltage cathode materials for sodium-ion batteries. Empirical potential parameters derived from our softBV bond valence parameter set reproduce experimental unit-cell parameters. Migration energy barrier calculations based on both these empirical and on ab initio approaches consistently show a strongly anisotropic and fairly fast Na+ ion mobility along partially occupied Na(3) channels in the c-direction. Nominally fully occupied Na(1) sites are attached to these paths with a moderate activation energy as sources of mobile ions. At elevated temperatures separate parallel Na(2) channels contribute to the ionic conductivity. As such one-dimensional pathways are highly vulnerable to blocking by structural defects, the experimentally observed favourable rate performance can only be understood as a consequence of cross-linking of the channels to a more robust higher-dimensional migration pathway network. Our static and dynamic bond valence pathway models for representative local structure models reveal that this cross-linking is achieved by the iron deficiency of the compound: iron vacancies act as low-lying interstitial sites that can be reached from both types of channels with moderate activation energies. Structural relaxations around the vacancies however reduce the sodium mobility along the channels. An analogous dual effect of blocking migration along the channels and promoting perpendicular migration would result from Na+/Fe2+ antisite defects. Hence, further new alluaudite type transition metal sulphates can only be expected to yield a high rate performance, if their synthesis ensures the presence of a comparable transition metal sub-stoichiometry and/or a suitably tailored concentration of sodium/transition metal antisite defects.

 Please wait while we load your content...
Please wait while we load your content...