The role of chemisorbed hydroxyl species in alkaline electrocatalysis of glycerol on gold†
Abstract
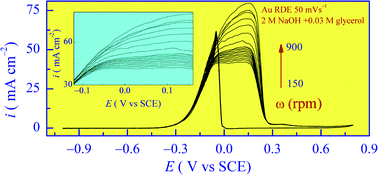
The mechanism of energy conversion in a direct glycerol fuel cell (DGFC) is governed by the anode supported heterogeneous steps of glycerol electro-oxidation. In aerated alkaline electrolytes, glycerol also participates in a base catalyzed process, which can release certain species mixing with the anode catalyzed surface products. As a result, selective probing of the surface catalytic reactions involving such systems can be difficult. The present work addresses this issue for a gold anode by using the analytical capability of cyclic voltammetry (CV). In addition, surface plasmon resonance measurements are used to optically probe the adsorption characteristics of the electrolyte species. The net exchange current of the oxidation process and the transfer coefficient of the rate determining step are evaluated by analyzing the CV data. The interfacial reactions and their products on Au are identified by measuring the number of electrons released during the electro-oxidation of glycerol. The results indicate that these reactions are facilitated by the surface bound hydroxyl species on Au (chemisorbed OH− and faradaically formed Au–OH). By comparing the findings for stationary and rotating electrodes, it is shown that, convective mass transport is critical to maintaining efficient progression of the consecutive oxidation steps of glycerol. In the absence of hydrodynamic support, the main surface products of glycerol oxidation appear to be glyceraldehyde, glycerate and malonate, formed through a net six-electron route. In the presence of controlled convection, a ten-electron process is activated, where mesaxolate is the likely additional product.

 Please wait while we load your content...
Please wait while we load your content...