Separating the contributions of the volume change upon mixing, permittivity contrast and molecular interactions in the excess relative permittivity of liquid mixtures
Abstract
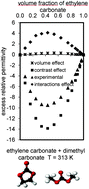
The excess relative permittivity of binary systems is separated into three parts. The excess molar volume is the basis for estimating the volume-change contribution. It is proposed to evaluate the electrical permittivity of liquid mixtures, which is solely due to the composition and the relative permittivities of pure components, named permittivity contrast contribution, using the classic local field approach in the case of point-dipoles contained in Lorentz's spherical cavities embedded in the corresponding ideal mixture. The effect of molecular interactions is simply estimated by the difference required to make up experimental excess relative permittivities. This analysis has been applied to 16 binary aqueous organic and organic–organic systems and the estimated values for the contribution of molecular interactions provide interesting insights into the molecular arrangement of these liquid mixtures and the suitability of solvents for determining solute dipole moments.

 Please wait while we load your content...
Please wait while we load your content...