Bimetallic dimers adsorbed on a defect-free MgO(001) surface: bonding, structure and reactivity
Abstract
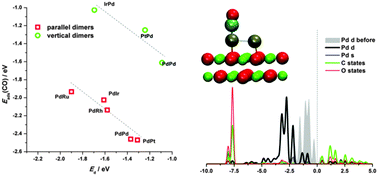
A large number of computational studies have been devoted to the investigation of monometallic clusters supported by MgO. However, in practice, catalysis shows that multicomponent catalytic systems often win in catalytic performance over single component systems. In this study, the geometrical and electronic structure, stability and chemisorption properties of M1M2 metal dimers (M1, M2 = Ru, Rh, Pd, Ir, Pt) supported by defect free MgO(001) have been investigated in the framework of density functional theory. The oxygen sites of MgO(001) are the preferred adsorption sites for all the studied clusters, the majority of them adsorbing parallel to the surface with metal atoms attached to two surface oxygen atoms. The energetics of M1M2 + MgO(001) formation shows that the adsorption complexes are stable and benefit from metal–oxygen and metal–metal interaction. The chemisorption properties of Pd and Pt atoms in PdM2 and PtM2 dimers are studied using CO as a probe molecule. A linear relationship between the CO chemisorption and the d-band center position of the reacting atom in the dimer is observed, extending the d-band center model to the case of highly under-coordinated metal atoms supported by a non-conductive material.

 Please wait while we load your content...
Please wait while we load your content...