Calcium peroxide from ambient to high pressures†
Abstract
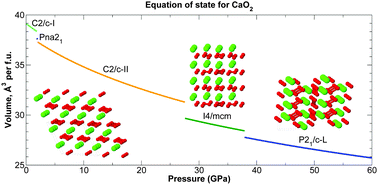
Structures of calcium peroxide (CaO2) are investigated in the pressure range 0–200 GPa using the ab initio random structure searching (AIRSS) method and density functional theory (DFT) calculations. At 0 GPa, there are several CaO2 structures very close in enthalpy, with the ground-state structure dependent on the choice of exchange–correlation functional. Further stable structures for CaO2 with C2/c, I4/mcm and P21/c symmetries emerge at pressures below 40 GPa. These phases are thermodynamically stable against decomposition into CaO and O2. The stability of CaO2 with respect to decomposition increases with pressure, with peak stability occurring at the CaO B1–B2 phase transition at 65 GPa. Phonon calculations using the quasiharmonic approximation show that CaO2 is a stable oxide of calcium at mantle temperatures and pressures, highlighting a possible role for CaO2 in planetary geochemistry. We sketch the phase diagram for CaO2, and find at least five new stable phases in the pressure–temperature ranges 0 ≤ P ≤ 60 GPa, 0 ≤ T ≤ 600 K, including two new candidates for the zero-pressure ground state structure.

 Please wait while we load your content...
Please wait while we load your content...