Parallel mechanisms of polypyrrole self-discharge in aqueous media
Abstract
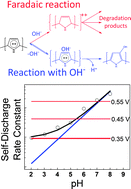
In this report we investigate the self-discharge in a positively charged polypyrrole–cellulose composite material in water solution. Rate constants for the self-discharge reaction are determined by potential step methods and their dependence on pH, temperature and applied potential are reported. Based on the results, we propose that two fundamentally different self-discharge mechanisms operate in parallel; one of faradaic origin with a rate constant increasing exponentially with applied potential and one mechanism comprising an initial reaction of the charged polymer with hydroxide ions. The second mechanism dominates at high pH as the rate constant for this reaction increases exponentially with pH whilst the faradaic reaction dominates at low pH. With this report we hope to shed light on the complex and elusive nature of self-discharge in conducting polymers to serve as guidance for the construction of electrical energy storage devices with conducting polymer components.

 Please wait while we load your content...
Please wait while we load your content...