Metal–organic Kagome lattices M3(2,3,6,7,10,11-hexaiminotriphenylene)2 (M = Ni and Cu): from semiconducting to metallic by metal substitution†
Abstract
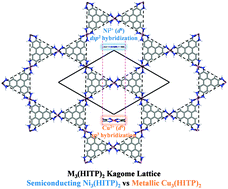
Motivated by recent experimental synthesis of a semiconducting metal–organic graphene analogue (J. Am. Chem. Soc., 2014, 136, 8859), i.e., Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2 [Ni3(HITP)2], a new Kagome lattice, Cu3(HITP)2, is designed by substituting the coordination of Ni by Cu. Such substitution results in interesting changes in electronic properties of the M3(HITP)2 bulk and two-dimensional (2D) sheets. In Ni3(HITP)2, each Ni atom adopts the dsp2 hybridization, forming a perfect 2D conjugation, whereas in Cu3(HITP)2, each Cu atom adopts the sp3 hybridization, resulting in a distorted 2D sheet. The M3(HITP)2 bulks, assembled from M3(HITP)2 sheets via both strong π–π interaction and weak metal–metal interaction, are metallic. However, the 2D Ni3(HITP)2 sheet is a semiconductor with a narrow band gap whereas the 2D Cu3(HITP)2 sheet is a metal. Remarkably, both the 2D M3(HITP)2 Kagome lattices possess Dirac bands in the vicinity of the Fermi level. Additional ab initio molecular dynamics simulations show that both sheets exhibit high thermal stability at elevated temperatures. Our theoretical study offers new insights into tunability of electronic properties for the 2D metal–organic frameworks (MOFs).

 Please wait while we load your content...
Please wait while we load your content...