Prediction of enhanced solvent-induced enantioselectivity for a ring opening with a bifurcating reaction path†
Abstract
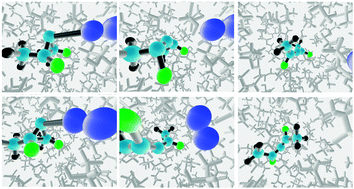
Classical molecular dynamics simulations are reported for the deazetisation and ring opening of meso-2,3-difluoro-2,3-dimethyldiazocyclopropane in three solvents: CHCl3, CHFClBr and CH3CH(OH)CF3 (TFIPA). The achiral reactant leads to enantiomeric allene products, and the question addressed in the study is whether either of the chiral, enantiomerically pure solvents can induce significant enantiomeric excess in the products. The direct dynamics calculations use an empirical valence bond potential for the solute, with empirical parameters optimised against M06-2X/cc-pVTZ density functional results. The results reveal that the exothermic N2 loss and ring opening promote transient strong solvent–solute interactions within the first ∼100 fs of the reaction. Because of the bifurcating reaction path, these interactions occur at time when the “decision” about which enantiomer of the product to form has yet to be made (at least for many of the trajectories). Hence, it is possible in principle that the solvent could exert a larger-than-normal influence on the course of the reaction. In fact, the results reveal no such effect for CHFClBr but do predict that TFIPA should induce 15.2 ± 2.1% enantiomeric excess. This is roughly an order of magnitude larger than solvent-induced enantiomeric excesses found experimentally in reactions where the conversion of reactant(s) to enantiomeric products occur over separate transition states.
- This article is part of the themed collections: PCCP Emerging Investigator Lectureship Award Winners and Bunsentagung 2015: Solvation Science

 Please wait while we load your content...
Please wait while we load your content...