An experimental and theoretical study of the photoisomerization and thermal reversion on 5-arylmethylene-2-thioxoimidazolidin-4-one†
Abstract
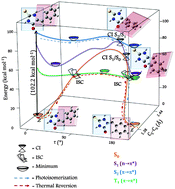
Unraveling the photochemical behaviour of the green fluorescent protein chromophore has lately attracted widespread attention among scientists. In this paper we present the study of the photochemical isomerization Z → E and the back reaction of the chromophore analog, 5-arylmethylene-2- thioxoimidazolidin-4-one. Experimental results are supported with ab initio calculations at the DFT, (B3LYP/6-31+g(d,p)), TD-DFT (B3LYP/6-311++g(3df,3pd)) and CASSCF levels. A first excitation to the S2 state, where the isomerization occurs, is proposed followed by two conical intersections to S1 and S0 respectively. Three different mechanisms were analyzed for thermal reversion, concluding that the preferred channel involves an intersystem crossing between the S0 and T1 states with the formation of a biradical.

 Please wait while we load your content...
Please wait while we load your content...