Mechanistic investigation of ion migration in Na3V2(PO4)2F3 hybrid-ion batteries
Abstract
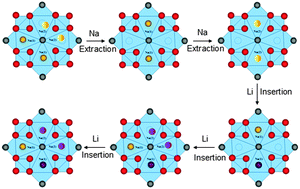
The ion-migration mechanism of Na3V2(PO4)2F3 is investigated in Na3V2(PO4)2F3–Li hybrid-ion batteries for the first time through a combined computational and experimental study. There are two Na sites namely Na(1) and Na(2) in Na3V2(PO4)2F3, and the Na ions at Na(2) sites with 0.5 occupation likely extract earlier to form Na2V2(PO4)2F3. The structural reorganisation is suggested to make a stable configuration of the remaining ions at the centre of Na(1) sites. After the extraction of the second Na ion, the last ion prefers to change occupation from 1 to 0.5 to occupy two Na(2) sites. The insertion of predominant Li ions also should undergo structural reorganization when the first Li ion inserts into the centre of Na(1) site theoretically forming NaLiV2(PO4)2F3, and the second ion inserts into two Na(2) sites to form NaLi2V2(PO4)2F3. More than a 0.3 Li ion insertion would take place in the applied voltage range by increasing the number of sites occupied rather than occupy the vacancy in triangular prismatic sites. An improved solution-based carbothermal reduction methodology makes Na3V2(PO4)2F3 exhibit excellent C-rate and cycling performances, of which the Li-inserted voltage is evaluated by first principles calculations.

 Please wait while we load your content...
Please wait while we load your content...