A look into amyloid formation by transthyretin: aggregation pathway and a novel kinetic model†
Abstract
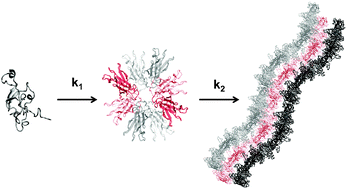
The aggregation of proteins into insoluble amyloid fibrils is the hallmark of many, highly debilitating, human pathologies such as Alzheimer's or Parkinson's disease. Transthyretin (TTR) is a homotetrameric protein implicated in several amyloidoses like Senile Systemic Amyloidosis (SSA), Familial Amyloid Polyneuropathy (FAP), Familial Amyloid Cardiomyopathy (FAC), and the rare Central Nervous System selective Amyloidosis (CNSA). In this work, we have investigated the kinetics of TTR aggregation into amyloid fibrils produced by the addition of NaCl to acid-unfolded TTR monomers and we propose a mathematically simple kinetic mechanism to analyse the aggregation kinetics of TTR. We have conducted circular dichroism, intrinsic tryptophan fluorescence and thioflavin-T emission experiments to follow the conformational changes accompanying amyloid formation at different TTR concentrations. Kinetic traces were adjusted to a two-step model with the first step being second-order and the second being unimolecular. The molecular species present in the pathway of TTR oligomerization were characterized by size exclusion chromatography coupled to multi-angle light scattering and by transmission electron microscopy. The results show the transient accumulation of oligomers composed of 6 to 10 monomers in agreement with reports suggesting that these oligomers may be the causative agent of cell toxicity. The results obtained may prove to be useful in understanding the mode of action of different compounds in preventing fibril formation and, therefore, in designing new drugs against TTR amyloidosis.


 Please wait while we load your content...
Please wait while we load your content...