Predicting the voltage dependence of interfacial electrochemical processes at lithium-intercalated graphite edge planes†
Abstract
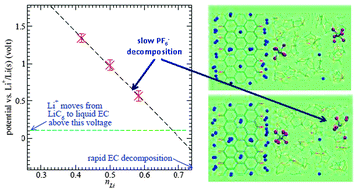
The applied potential governs lithium-intercalation and electrode passivation reactions in lithium ion batteries, but are challenging to calibrate in condensed phase DFT calculations. In this work, the “anode potential” of charge-neutral lithium-intercalated graphite (LiC6) with oxidized edge planes is computed as a function of Li-content (nLi) at edge planes, using ab initio molecular dynamics (AIMD), a previously introduced Li+ transfer free energy method, and the experimental Li+/Li(s) value as reference. The voltage assignments are corroborated using explicit electron transfer from fluoroethylene carbonate radical anion markers. PF6− is shown to decompose electrochemically (i.e., not just thermally) at low potentials imposed by our voltage calibration technique. We demonstrate that excess electrons reside in localized states-in-the-gap in the organic carbonate liquid region, which is not semiconductor-like (band-state-like) as widely assumed in the literature.

 Please wait while we load your content...
Please wait while we load your content...