Unraveling the degradation of artificial amide bonds in nylon oligomer hydrolase: from induced-fit to acylation processes†
Abstract
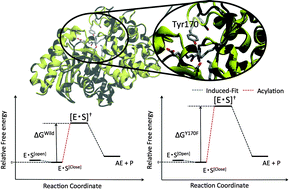
To elucidate how the nylon oligomer hydrolase (NylB) acquires its peculiar degradation activity towards non-biological amide bonds, we inspected the underlying enzymatic processes going from the induced-fit upon substrate binding to acylation. Specifically we investigated the mutational effects of two mutants, Y170F and D181G, indicated in former experiments as crucial systems because of their specific amino acid residues. Therefore, by adopting first-principles molecular dynamics complemented with metadynamics we provide a detailed insight into the underlying acylation mechanism. Our results show that while in the wild type (WT) the Tyr170 residue points the NH group towards the proton-acceptor site of an artificial amide bond, hence ready to react, in the Y170F this does not occur. The reason is ascribed to the absence of Tyr170 in the mutant, which is replaced by phenylalanine, which is unable to form hydrogen bond with the amide bond; thus, resulting in an increase in the activation barrier of more than 10 kcal mol−1. Nonetheless, despite the lack of hydrogen bonding between the Y170F and the substrate, the highest free energy barrier for the induced-fit is similar to that of WT. This seems to suggest that in the induced-fit process, kinetics is little affected by the mutation. On the basis of additional structural homology analyses on the enzymes of the same family, we suggest that natural selection is responsible for the development of the peculiar hydrolytic activity of Arthrobacter sp. KI72.


 Please wait while we load your content...
Please wait while we load your content...