Classical nucleation theory of homogeneous freezing of water: thermodynamic and kinetic parameters
Abstract
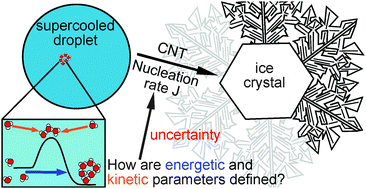
The probability of homogeneous ice nucleation under a set of ambient conditions can be described by nucleation rates using the theoretical framework of Classical Nucleation Theory (CNT). This framework consists of kinetic and thermodynamic parameters, of which three are not well-defined (namely the interfacial tension between ice and water, the activation energy and the prefactor), so that any CNT-based parameterization of homogeneous ice formation is less well-constrained than desired for modeling applications. Different approaches to estimate the thermodynamic and kinetic parameters of CNT are reviewed in this paper and the sensitivity of the calculated nucleation rate to the choice of parameters is investigated. We show that nucleation rates are very sensitive to this choice. The sensitivity is governed by one parameter – the interfacial tension between ice and water, which determines the energetic barrier of the nucleation process. The calculated nucleation rate can differ by more than 25 orders of magnitude depending on the choice of parameterization for this parameter. The second most important parameter is the activation energy of the nucleation process. It can lead to a variation of 16 orders of magnitude. By estimating the nucleation rate from a collection of droplet freezing experiments from the literature, the dependence of these two parameters on temperature is narrowed down. It can be seen that the temperature behavior of these two parameters assumed in the literature does not match with the predicted nucleation rates from the fit in most cases. Moreover a comparison of all possible combinations of theoretical parameterizations of the dominant two free parameters shows that one combination fits the fitted nucleation rates best, which is a description of the interfacial tension coming from a molecular model [Reinhardt and Doye, J. Chem. Phys., 2013, 139, 096102] in combination with the activation energy derived from self-diffusion measurements [Zobrist et al., J. Phys. Chem. C, 2007, 111, 2149]. However, some fundamental understanding of the processes is still missing. Further research in future might help to tackle this problem. The most important questions, which need to be answered to constrain CNT, are raised in this study.

 Please wait while we load your content...
Please wait while we load your content...