A new working mode for molecular springs: water intrusion induced by cooling and associated isobaric heat capacity change of a {ZIF-8 + water} system†
Abstract
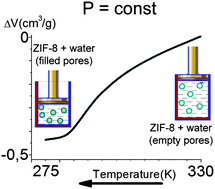
Hydrophobic microporous metal–organic framework ZIF-8 combined with water forms a molecular spring (MS), which by the forced intrusion of water into the pores and its spontaneous extrusion can store and restore large amounts of mechanical and thermal energy. Using scanning transitiometry technique, we demonstrate that the simultaneous effect of temperature and pressure on the porosity of ZIF-8 leads to a non-standard temperature dependence of intrusion and extrusion pressures of MS, which allows to provoke water intrusion into the hydrophobic pores of ZIF-8 by decreasing the temperature of the system under constant pressure. A remarkably strong effect of intrusion on the isobaric heat-capacity of {ZIF-8 + water} MS is discovered.


 Please wait while we load your content...
Please wait while we load your content...