Coverage-dependent thermodynamic analysis of the formation of water and hydrogen peroxide on a platinum model catalyst†
Abstract
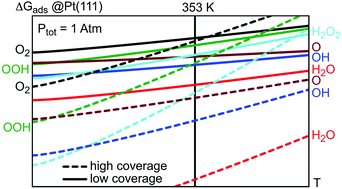
Understanding the selectivity of the oxygen reduction reaction, especially the formation of water versus hydrogen peroxide in fuel cells, is an ongoing challenge in electrochemistry, surface science and catalysis. In this study, we propose a comprehensive thermodynamic analysis of the reaction intermediates for the formation of water on Pt(111). Density functional theory calculations of all the elementary steps linking hydroxyl and hydroperoxyl surface species with water and hydrogen peroxide have been performed at low (1/12 ML, ML = monolayer) and high (1/4 ML) coverages. The reaction energy variation for the two competing elementary events (molecular oxygen dissociation and hydroperoxyl formation) is strongly coverage-dependent. For the direct dissociation, an increase is observed at low coverage with respect to the usual high coverage picture. The stability of the reaction intermediates is investigated from thermodynamic diagrams. At 353 K and a total pressure of 1 atm, water and hydroxyl surface species are expected to compete for adsorption on Pt(111).

 Please wait while we load your content...
Please wait while we load your content...