The mechanism of the Pd-catalyzed formation of coumarins: a theoretical study†
Abstract
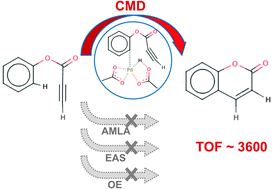
The mechanism of the formation of coumarins via the Pd-catalyzed intramolecular hydroarylation of the C–C triple bond is elucidated computationally, in corroboration with experimental data. It is shown that the reaction follows the concerted metalation–deprotonation (CMD) mechanism. The typically suspected mechanisms of ambiphilic metal ligand activation (AMLA), electrophilic aromatic substitution (EAS), and oxidative addition (OA) are suggested to be non-competitive, based on predicted conformations and energetics. Two forms of the Pd catalysts are used: Pd(OAc)2, and Pd(TFA)2. The predicted activation free energy barrier for the TFA-based catalyst is lower, both in the gas phase and in the CH2Cl2 solvent, in agreement with the experimental observations. Adding electron-withdrawing groups to the catalyst assists the first and rate-limiting step of the reaction, the deprotonation of the aromatic ring, as understood through charge analysis.

 Please wait while we load your content...
Please wait while we load your content...