The roughness of the protein energy landscape results in anomalous diffusion of the polypeptide backbone†
Abstract
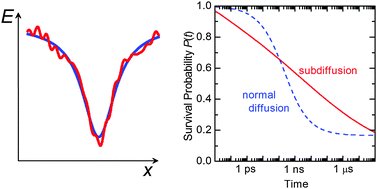
Although protein folding is often described by motion on a funnel-shaped overall topology of the energy landscape, the many local interactions that can occur result in considerable landscape roughness which slows folding by increasing internal friction. Recent experimental results have brought to light that this roughness also causes unusual diffusional behaviour of the backbone of an unfolded protein, i.e. the relative motion of protein sections cannot be described by the normal diffusion equation, but shows strongly subdiffusional behaviour with a nonlinear time dependence of the mean square displacement, 〈r2(t)〉 ∝ tα (α ≪ 1). This results in significantly slower configurational equilibration than had been assumed hitherto. Analysis of the results also allows quantification of the energy landscape roughness, i.e. the root-mean-squared depth of local minima, yielding a value of 4–5kBT for a typical small protein.

 Please wait while we load your content...
Please wait while we load your content...