Rare stoichiometry of carboxyl–carboxylate benzbetaine complexes: in vitro versus in silico†
Abstract
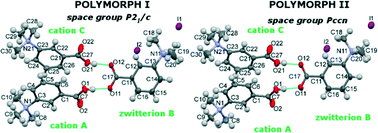
The first betaine–mineral acid complex of 3 : 2 stoichiometry, as well as a 1 : 1 complex common for this class of compounds, has been obtained. Crystalline [di-(3-trimethylammonium-benzoic acid)–(3-trimethylammonium-benzoate)]diiodide (1) is the only betaine–mineral acid 3 : 2 complex obtained so far. Two concomitant polymorphs of this 3 : 2 complex, 1-I and 1-II, have been isolated and characterized by X-ray diffraction and FTIR spectroscopy. Polymorphs 1-I, of monoclinic space group P21/c, and 1-II, of orthorhombic space group Pccn, have very similar lattices and aggregates of one zwitterionic molecule and two cations O–H⋯O bonded into a 3-membered catemeric carboxyl–carboxylate–carboxyl interval, but polymorph 1-II dissolves better in methanol than 1-I. Alternative hypothetical aggregates of 3-trimethylammonium-benzoate hydroiodide 1 : 1, 2 : 1 and 3 : 2 complexes optimized at the B3LYP/6-31G(d,p) level of theory suggest that the betaine-to-acid 3 : 2 stoichiometry is favoured at the stage of small cluster aggregation and not due to the complex crystal packing preferences.

 Please wait while we load your content...
Please wait while we load your content...