Oxime synthons in the salts and cocrystals of quinoline-4-carbaldoxime for non-covalent synthesis†
Abstract
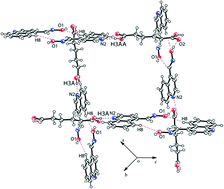
The oxime–quinoline R22(8)-type heterosynthons in self-assemblies of quinoline-4-carbaldoxime change to form R22(14)-type oxime–oxime homosynthons and quinoline–carboxylic acid heterosynthons in cocrystals upon interactions with dicarboxylic acids such as adipic, succinic and fumaric acids. The general trend observed in the formation similar oxime–oxime homosynthons of these cocrystals is useful to demarcate these structures to have obtained through predesigned non-covalent synthesis. On the other hand, the maleate salt of quinoline-4-carbaldoxime has two self-interacting maleate anions flanked by two quinolinium-4-carbaldoxime cations, whereas the oxalate salt has the dianions flanked by two cations. Among the salts of acids like nitric acid and hydrochloric acid, the nitrate salt has nitrate ions bridging two quinolinium-4-carbaldoxime cations to form cyclic motifs, whereas the self-assembly of the chloride salt has chloride–water chains formed through hydrogen bonds. The self-assembly of the chloride salt has conventional R22(8)-type oxime–oxime homosynthons.

 Please wait while we load your content...
Please wait while we load your content...