Time-resolved in situ studies on the formation mechanism of iron oxide nanoparticles using combined fast-XANES and SAXS†
Abstract
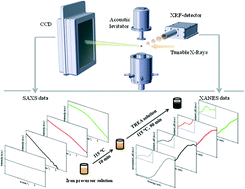
The reaction of iron chlorides with an alkaline reagent is one of the most prominent methods for the synthesis of iron oxide nanoparticles. We studied the particle formation mechanism using triethanolamine as reactant and stabilizing agent. In situ fast-X-ray absorption near edge spectroscopy and small-angle X-ray scattering provide information on the oxidation state and the structural information at the same time. In situ data were complemented by ex situ transmission electron microscopy, wide-angle X-ray scattering and Raman analysis of the formed nanoparticles. The formation of maghemite nanoparticles (γ-Fe2O3) from ferric and ferrous chloride was investigated. Prior to the formation of these nanoparticles, the formation and conversion of intermediate phases (akaganeite, iron(II, III) hydroxides) was observed which undergoes a morphological and structural collapse. The thus formed small magnetite nanoparticles (Fe3O4) grow further and convert to maghemite with increasing reaction time.

 Please wait while we load your content...
Please wait while we load your content...