Morphology evolution of α-Fe2O3 controlled via incorporation of alkaline earth metal ions
Abstract
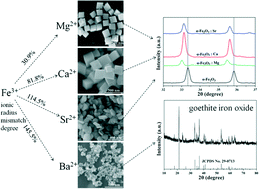
Hematite (α-Fe2O3) crystals with different morphologies were successfully fabricated via an environmental friendly hydrothermal route by applying alkaline earth metal ions along the row from Mg2+ to Ba2+ ions as structure-directing agents without employing any surfactants or templates. It was found that the ionic radii mismatch degree between the alkaline earth metal ions and Fe3+ ions played a vital role in determining the crystalline phase and morphology of the iron oxide. Pure α-Fe2O3 crystals were created with Mg2+, Ca2+ and Sr2+ ions (30.9%, 81.8% and 114.5% ionic radii mismatch degree with that of Fe3+) as additives while goethite iron oxide nanoparticles were obtained with Ba2+ ions (145.5% ionic radius mismatch degree with that of Fe3+) as additives. Mg2+ ion-induced α-Fe2O3 crystals had a quasi-cube structure with a rough surface while Ca2+-induced α-Fe2O3 crystals had a quasi-cube structure with a very smooth surface, and the edge length of which is much smaller than that of the Mg2+ ion-controlled α-Fe2O3 crystals. However, when Sr2+ ions were introduced into the reaction system, the product tended to have plate-like structures. The effects of alkaline earth metal ions on the crystal growth rate and final morphology through anisotropic incorporation were proposed in this paper. Moreover, the effects of incorporation of alkaline earth metal ions on the magnetic properties of α-Fe2O3 crystals were investigated.

 Please wait while we load your content...
Please wait while we load your content...