The effects of molecular flexibility and substituents on conformational polymorphism in a series of 2,5-diamino-3,6-dicyanopyrazine dyes with highly flexible groups†
Abstract
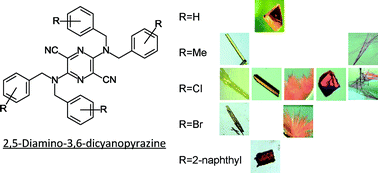
Conformational polymorphism of a series of 2,5-bis(N,N-dibenzylamino)-3,6-dicyanopyrazine dyes, with high molecular flexibility in a non-hydrogen-bonding system, is described. Three derivatives having a substituent at the 4-position of the benzyl groups exhibited several crystal forms with different colours (red, orange, and yellow), whereas a single form was found in the unsubstituted benzyl derivative and the derivative containing 2-naphthylmethyl groups. The effects of conformational flexibility and the substituents on conformational polymorphism were evaluated through structural, energetic, and statistical analyses based on all of the observed crystal structures. They were divided into four conformational groups according to their conformational similarities, and the major difference among the groups was attributed to the amino geometry. Statistical research using the Cambridge Structure Database suggested that a dibenzylamino group connected to an aromatic ring had a suitable potential surface for the occurrence of conformational polymorphs, and had at least two potential wells with respect to the amino geometry. This result revealed that the flexibility of the dibenzylamino group in the pyrazine derivatives enabled the formation of various molecular shapes. However, the preference of occurrence of polymorphs was different in these derivatives. Crystal structure analysis indicated that the similarity in the molecular shapes was significant for the closed packing in the thermally stable forms, whereas the terminal substituents on the dibenzyl groups played an important role in the crystal structures of the thermally metastable forms via the formation of weak interactions such as halogen interactions.

 Please wait while we load your content...
Please wait while we load your content...