Enantiomeric poly(d-lactide) with a higher melting point served as a significant nucleating agent for poly(l-lactide)†
Abstract
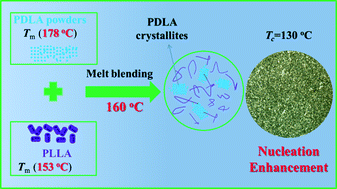
In the present work, a new approach to significantly accelerate the crystallization of poly(L-lactide) (PLLA) by utilizing poly(D-lactide) (PDLA) crystallites is presented. In particular, a PDLA with a high melting point (25 °C higher than that of PLLA) and high crystallinity (hPDLA) was introduced into the PLLA matrix via melt blending at a temperature between the melting points of PLLA and hPDLA. It was proved that the hPDLA crystallites were not melted and were instead reserved in the PLLA matrix. Results showed that a small amount of hPDLA crystallites can largely elevate the crystallization temperature, reduce the crystallization half-time and promote the crystallization rate of PLLA. In situ polarizing microscope observations showed that the great improvement of the crystallization rate was caused by the excellent nucleation effect of the hPDLA crystallites. Wide-angle X-ray diffraction results showed that the crystalline structures of the PLLA crystallites and hPDLA crystallites showed very good lattice match, which results in a largely reduced energy barrier for nucleation and increased crystallization kinetics.

 Please wait while we load your content...
Please wait while we load your content...