Reversible adsorption and separation of chlorocarbons and BTEX based on Cu(ii)-metal organic framework†
Abstract
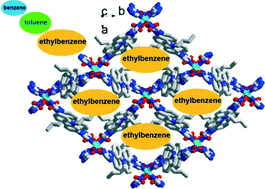
A new non-interpenetrating 2D Cu(II)-metal organic framework has been successfully synthesized from the carbazole-bridging organic ligand L and Cu(NO3)2 in solution. The CuL2(NO3)2 framework contains square-like channels and the n-butyl groups on L face toward the channel center to form the typical hydrophobic pores. In addition, the reported CuL2(NO3)2 host can reversibly upload various VOCs such as CH2Cl2, CHCl3 and BTEX (benzene, toluene, ethylbenzene, o-xylene, m-xylene, and p-xylene) under ambient conditions without loss of framework integrity. Furthermore, it is able to effectively separate CH2Cl2 from CHCl3, benzene from toluene/ethylbenzene/xylene and toluene from ethylbenzene/xylene in the liquid phase. The selectivity for chlorocarbons is derived from the substrate polarity, while the host–guest hydrophobic interaction might be the dominating factor for BTEX affinity.

 Please wait while we load your content...
Please wait while we load your content...