Crystalline inclusion properties of new pyridine and thiophene modified wheel-and-axle diol hosts†
Abstract
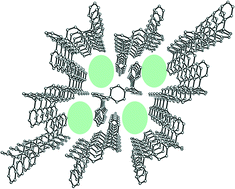
Structural extension of the ‘wheel-and-axle’ host concept has given rise to the design of a new type of host derivative (1–3) featuring two di(pyrid-2-yl)hydroxymethyl or di(thien-2-yl)hydroxymethyl moieties attached to a central phenylene unit, i.e. having the phenyl groups of a well-known model compound (Ph) substituted by heteroaromatics. Syntheses of the new derivative compounds are described, and the capability to form crystalline inclusions obtained from solvent solution compared to the prototype molecule is studied, including X-ray diffraction analysis of relevant crystal structures. Sorption measurements of the compounds as solid receptor films coated on a quartz crystal microbalance considering a variety of solvent vapors have been performed, showing potential application as mass sensitive sensor materials.

 Please wait while we load your content...
Please wait while we load your content...