Hopping intermittent contact-scanning electrochemical microscopy (HIC-SECM) as a new local dissolution kinetic probe: application to salicylic acid dissolution in aqueous solution
Abstract
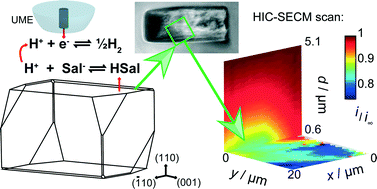
Dissolution kinetics of the (110) face of salicylic acid in aqueous solution is determined by hopping intermittent contact-scanning electrochemical microscopy (HIC-SECM) using a 2.5 μm diameter platinum ultramicroelectrode (UME). The method operates by translating the probe UME towards the surface at a series of positions across the crystal and inducing dissolution via the reduction of protons to hydrogen, which titrates the weak acid and promotes the dissolution reaction, but only when the UME is close to the crystal. Most importantly, as dissolution is only briefly and transiently induced at each location, the initial dissolution kinetics of an as-grown single crystal surface can be measured, rather than a surface which has undergone significant dissolution (pitting), as in other techniques. Mass transport and kinetics in the system are modelled using finite element method simulations which allows dissolution rate constants to be evaluated. It is found that the kinetics of an ‘as-grown’ crystal are much slower than for a surface that has undergone partial bulk dissolution (mimicking conventional techniques), which can be attributed to a dramatic change in surface morphology as identified by atomic force microscopy (AFM). The ‘as-grown’ (110) surface presents extended terrace structures to the solution which evidently dissolve slowly, whereas a partially dissolved surface has extensive etch features and step sites which greatly enhance dissolution kinetics. This means that crystals such as salicylic acid will show time-dependent dissolution kinetics (fluxes) that are strongly dependent on crystal history, and this needs to be taken into account to fully understand dissolution.

 Please wait while we load your content...
Please wait while we load your content...