Manganese sesquioxide to trimanganese tetroxide hierarchical hollow nanostructures: effect of gadolinium on structural, thermal, optical and magnetic properties†
Abstract
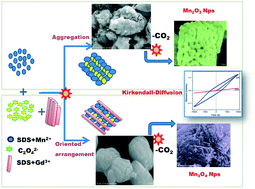
Various hollow manganese oxide (bixbyite Mn2O3 and hausmannite Mn3O4) nanoparticles (NPs) with different morphologies were obtained from a single precursor, manganese oxalate (MnC2O4). To synthesize a Mn3O4 stacked nanostructure rather than coral-like Mn2O3 nanospheres, as synthesized MnC2O4 was thermally decomposed at 700 °C in the presence of Gd3+, through the oriented arrangement mechanism. The formation process and structural variation arising from varying the thermal treatment (450 °C and 700 °C) and the cationic dopant Gd3+ were analyzed by FTIR, TGA, and XRD. The unexpected size reduction, and significant physicochemical properties were analyzed using various techniques such as FESEM coupled with EDAX, HR-TEM, DRS-UV-vis, EPR, EIS and VSM. The addition of gadolinium induces particle size reduction and a phase transition from cubic Mn2O3 to tetragonal Mn3O4, which leads to the suppression of the electrical conductivity, and changes in the optical band gap. The prepared Mn3O4 nanocrystals exhibit ferromagnetic behavior below Tc ≈ 45 K and weak paramagnetic behavior at room temperature.

 Please wait while we load your content...
Please wait while we load your content...