Solid-state identity of 2-hydroxynicotinic acid and its polymorphism†
Abstract
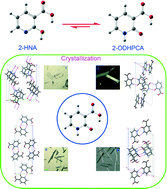
2-Hydroxynicotinic acid (2-HNA), a derivative of nicotinic acid, was found to exist in four polymorphs. In the solid state, 2-HNA is actually present as its tautomer, 2-oxo-1,2-dihydro-3-pyridinecarboxylic acid (2-ODHPCA). The polymorphism stems from distinctive packing arrangements of the molecules, and the formation of the distinct polymorphs can be affected by using various acidic additives. The thermal behaviors of the four polymorphs indicate that form I is the most stable at elevated temperature, form II converts into form I during heating, and forms III and IV transform into form II when heated. Theoretical studies showed that 2-ODHPCA is more energetically favored than 2-HNA. Condensed Fukui functions and the dual descriptor were used jointly to examine the local preference of hydrogen bonding in the crystal. Lattice energy calculations were conducted to further evaluate energetic properties of the system.
- This article is part of the themed collection: Polymorphism

 Please wait while we load your content...
Please wait while we load your content...