Entanglement in Co(ii) coordination networks: polycatenation from single net to 2-fold and 3-fold interpenetrated nets†
Abstract
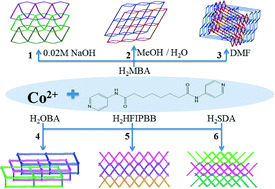
The synthesis, structures and properties of six coordination networks based on N,N′-di(4-pyridyl)suberoamide (L) and angular dicarboxylate ligands, {[Co(L)(MBA)]·2H2O}n (H2MBA = diphenylmethane-4,4′-dicarboxylic acid), 1, {[Co(L)0.5(MBA)]·CH3OH}n, 2, [Co(L)0.5(MBA)]n, 3, {[Co2(L)(OBA)2]·7CH3OH}n [H2OBA = 4,4′-oxybis(benzoic acid)], 4, {[Co(L)(hfipbb)]·2H2O}n [H2hfipbb = 4,4′-(hexafluoroisopropylidene)bis(benzoic acid)], 5, and {[Co(L)(SDA)]·H2O}n (H2SDA = 4,4′-sulfonyldibenzoic acid), 6, are reported. Complex 1 is a 1D → 2D polycatenane derived from the helical channels, whereas both complexes 2 and 3 show 2D layers with the (42·68·8·104)(4)2-2,6L1 topology, which are further entangled to form a 2-fold 2D → 2D interpenetration network and a 2D → 3D inclined polycatenation network, respectively, and complex 4 shows a 3-fold 3D → 3D interpenetration network with the (412·63)-pcu topology. The unusual topological features of 5 and 6 consist of 2- and 3-fold interpenetrated layers with the (44·62)-sql topology, which are further catenated to the two such adjacent sheets in parallel and uniform fashions to give 2D → 3D polycatenated networks. Complexes 1, 5 and 6 represent unique examples that the angular dicarboxylate ligands show significant effect on the degree of interpenetration of polycatenation coordination networks with interpenetrating modes. The CO2 capture is preferable to N2 and H2 in the gas sorption for the desolvated product of 4.

 Please wait while we load your content...
Please wait while we load your content...