Fluorinated azobenzenes with highly strained geometries for halogen bond-driven self-assembly in the solid state†
Abstract
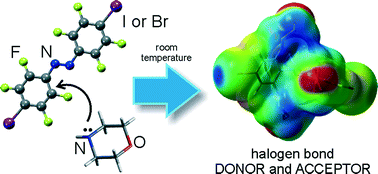
Attempted cocrystallisation of brominated and iodinated octafluoroazobenzene derivatives with morpholine led to the exhaustive replacement of fluorine substituents that are in ortho-positions to the azobenzene with sterically demanding morpholine groups. The resulting molecules exhibit a highly unusual strained conformation of the azobenzene unit, in which the terminal phenyl rings adopt a mutually nearly completely orthogonal orientation. Substitution of ortho-fluorine groups with N-morpholine fragments provides molecules with active halogen bond donor and acceptor sites that guide the molecular self-assembly in the solid state towards the formation of polymeric halogen-bonded chains.
- This article is part of the themed collection: International Year of Crystallography Celebration: North America

 Please wait while we load your content...
Please wait while we load your content...