Combining cycloisomerization with trienamine catalysis: a regiochemically flexible enantio- and diastereoselective synthesis of hexahydroindoles†
Abstract
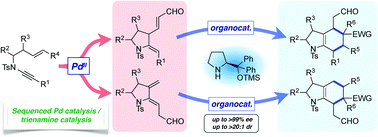
The synthesis of polysubstituted hexahydroindoles through trienamine-organocatalyzed cycloadditions of pyrrolidinyl dienals, prepared by palladium-catalyzed cycloisomerization, is reported. The cycloadditions of this novel class of dienals proceed with excellent levels of enantio- and diastereoselectivity, with the regioselectivity of cycloaddition with respect to the tethering ring readily tuned through design of the cycloisomerization substrate. This work culminates in the first examples of double-stereodifferentiating trienamine catalysis, where catalyst stereocontrol dominates facial selectivity in the cycloaddition, affording azacyclic products that are specifically functionalized at every position.

 Please wait while we load your content...
Please wait while we load your content...