The reductive P–P coupling of primary and secondary phosphines mediated by N-heterocyclic carbenes†
Abstract
The dehydrogenative coupling of primary and secondary phosphines with the N-heterocyclic carbene iPr2Im (1,3-di-isopropyl-imidazolin-2-ylidene) has been reported. The dehydrogenation of R2PH affords diphosphines R2P–PR2. The reaction of iPr2Im with ArPH2 leads to the formation of NHC phosphinidene adducts iPr2Im![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PAr and cyclic oligophosphines P4Ar4, P5Ar5 and P6Ar6, depending on the stoichiometry used. The NHC acts in these reactions as a phosphine activator and hydrogen acceptor.
PAr and cyclic oligophosphines P4Ar4, P5Ar5 and P6Ar6, depending on the stoichiometry used. The NHC acts in these reactions as a phosphine activator and hydrogen acceptor.
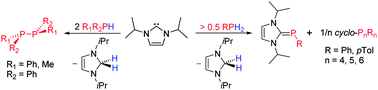

 Please wait while we load your content...
Please wait while we load your content...