Enantioselective synthesis of planar-chiral benzosiloloferrocenes by Rh-catalyzed intramolecular C–H silylation†
Abstract
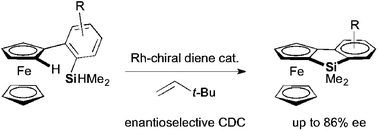
The first synthesis of planar-chiral benzosiloloferrocenes was achieved by the intramolecular reaction of 2-(dimethylhydrosilyl)arylferrocenes. The enantioselective cross dehydrogenative coupling of an sp2 C–H bond of ferrocene with a Si–H bond proceeded efficiently with the use of a Rh-chiral diene catalyst.

 Please wait while we load your content...
Please wait while we load your content...