A novel fluoro-chromogenic click reaction for the labelling of proteins and nanoparticles with near-IR theranostic agents†‡
Abstract
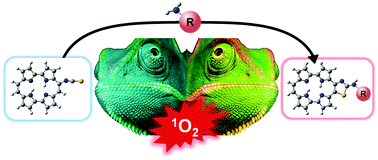
Reaction of porphycene isothiocyanates with primary and secondary amines leads to the formation of thiazolo[4,5-c]porphycenes, with a substantial shift in the absorption and fluorescence spectra. The conjugates show fluorescence in the near-infrared and are capable of photosensitizing the production of the cytotoxic species singlet oxygen.


 Please wait while we load your content...
Please wait while we load your content...