Palladium catalyzed Csp2–H activation for direct aryl hydroxylation: the unprecedented role of 1,4-dioxane as a source of hydroxyl radicals†
Abstract
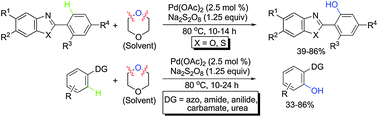
A novel strategy for direct aryl hydroxylation via Pd-catalysed Csp2–H activation through an unprecedented hydroxyl radical transfer from 1,4-dioxane, used as a solvent, is reported with bio relevant and sterically hindered heterocycles and various acyclic functionalities as versatile directing groups.

 Please wait while we load your content...
Please wait while we load your content...