Amyloid-β adopts a conserved, partially folded structure upon binding to zwitterionic lipid bilayers prior to amyloid formation†
Abstract
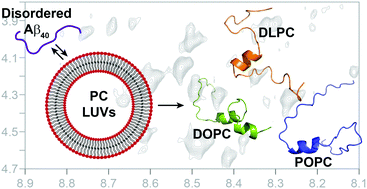
Aggregation at the neuronal cell membrane's lipid bilayer surface is implicated in amyloid-β (Aβ) toxicity associated with Alzheimer's disease; however, structural and mechanistic insights into the process remain scarce. We have identified a conserved binding mode of Aβ40 on lipid bilayer surfaces with a conserved helix containing the self-recognition site (K16-E22).

 Please wait while we load your content...
Please wait while we load your content...