Protonation of silylenol ether via excited state proton transfer catalysis†
Abstract
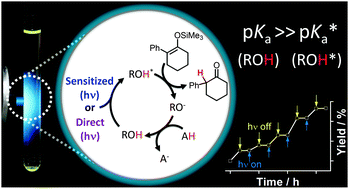
We demonstrate the photocatalytic protonation of a silylenol ether using 7-bromo-2-naphthol as an ESPT catalyst with phenol as the sacrificial proton source. Greater than 95% conversion is achieved with 1 mol% catalyst. The reaction cycle is dependent on the significantly increased acidity of the catalyst in the excited state as well as the long lifetime for the triplet excited state of 7-bromo-2-naphthol. The reaction does not occur in the absence of light (367 nm) and can readily be controlled by light intensity modulation. We also demonstrate that a 72% reaction yield can be obtained with unsubstituted naphthol as the catalyst by coupling triplet energy transfer, via a visible light absorbing (445 nm) sensitizer, into the catalytic cycle. These results open the door to an entirely new class of sensitized photocatalytic reactions that harness the excited state acidity of ESPT dyes.

 Please wait while we load your content...
Please wait while we load your content...