The synthesis and structure of a carbene-stabilized iminocarboranyl-boron(i) compound†
Abstract
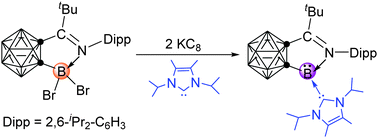
A carbene-stabilized iminocarboranylboron(I) compound has been synthesized and structurally characterized. Single-crystal X-ray analyses and DFT calculations show that the π back donation of the lone pair of electrons on the boron(I) center onto the π* orbital of the imine unit is crucial for removing the electron density of the boron center thereby stabilizing such species, in which the carbene serves solely as a σ donor. This work also demonstrates that imines play a similar role to that of carbenes in the stabilization of low valent boron compounds.

 Please wait while we load your content...
Please wait while we load your content...