H2 oxidation versus organic substrate oxidation in non-heme iron mediated reactions with H2O2†
Abstract
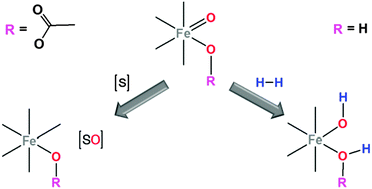
Herein we show that species generated upon reaction of α-[Fe(CF3SO3)2(BPMCN)] (BPMCN = N,N′-bis(2-pyridylmethyl)-trans-1,2-diaminocyclohexane) with H2O2 (putatively [FeV(O)(OH)(BPMCN)]) is able to efficiently oxidize H2 to H2O even in the presence of organic substrates, while species formed in the presence of acetic acid (putatively [FeV(O)(OAc)(BPMCN)]) prefer organic substrate oxidation over H2 activation. Mechanistic implications have been analysed with the aid of computational methods.

 Please wait while we load your content...
Please wait while we load your content...