Chrysaorenes: assembling coronoid hydrocarbons via the fold-in synthesis†
Abstract
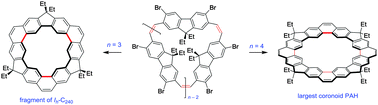
Two coronoid hydrocarbons, [3]- and [4]chrysaorene were synthesized from fluorenophane precursors using the fold-in strategy. [3]Chrysaorene is a bowl-shaped fragment of the C240-Ih fullerene, whereas [4]chrysaorene is planar and contains a unique large 24-membered internal ring. The chrysaorenes show geometry-dependent magnetic properties and are strongly fluorescent.

 Please wait while we load your content...
Please wait while we load your content...