Sterically geared tris-thioureas; transmembrane chloride transporters with unusual activity and accessibility†
Abstract
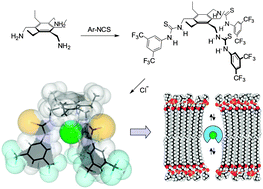
Tris-N-arylthioureas derived in one step from 1,3,5-tris(aminomethyl)-2,4,6-triethylbenzene are remarkably effective anion carriers. With optimised aryl substituents their activities come close to the best currently known, suggesting that they might find use as readily available standards in anion transport research.

 Please wait while we load your content...
Please wait while we load your content...